The IP 109 pill is a widely used prescription medication containing hydrocodone bitartrate (5 mg) and acetaminophen (325 mg). The primary purpose of IP 109 is for the management of moderate to severe pain, leveraging the synergistic effects of hydrocodone, an opioid analgesic, and acetaminophen, a non-opioid pain reliever. It is prescribed for short-term pain relief following surgery or injury and for certain types of chronic pain conditions. [1]
However, hydrocodone, a Schedule II controlled substance in the US, has significant potential for misuse. Hydrocodone is known to have euphoric effects at higher doses, making it appealing as a recreational drug and potentially leading to dependency.
Studies indicate that misuse of IP 109 can lead to addiction and overdose. [2] The acetaminophen component present in IP 109 is generally safe when it is used as directed, but it can lead to severe liver damage if it is consumed in excessive amounts. [3]
- IP 109 is a prescription pill containing hydrocodone bitartrate (5 mg) and acetaminophen (325 mg). It is primarily used for short-term relief of moderate to severe pain.
- Common side effects include drowsiness, dizziness, nausea, and constipation. Serious risks include respiratory depression, liver damage from excessive acetaminophen intake, dependency, and overdose.
- Take it only as prescribed, avoid exceeding six tablets in 24 hours, and do not combine IP 109 with alcohol or other acetaminophen-containing products or medicine. Store it securely to prevent misuse, and consult a doctor if pregnant, breastfeeding, or using other medications.

What is the IP 109 pill?
The IP 109 pill is a prescription medication that contains two active ingredients: hydrocodone bitartrate (5 mg) and acetaminophen (325 mg). It is primarily used for the management of moderate to severe pain, combining the effects of an opioid analgesic with a non-opioid pain reliever.
Hydrocodone bitartrate is a semi-synthetic opioid that works by binding to mu-opioid receptors in the central nervous system (CNS). This reduces pain perception and modifies the emotional and physical response to discomfort and pain. [4]
Acetaminophen, on the other hand, is a non-opioid analgesic and antipyretic. In other words, it alleviates pain and reduces fever. This is done by acetaminophen inhibiting the production of prostaglandins in the CNS and acting on the hypothalamus in the brain to regulate body temperature. [1]
The synergistic (enhancing) effects of hydrocodone and acetaminophen augment the pain-relieving properties, which makes IP 109 effective for managing acute pain conditions.
Identifying IP 109 pills
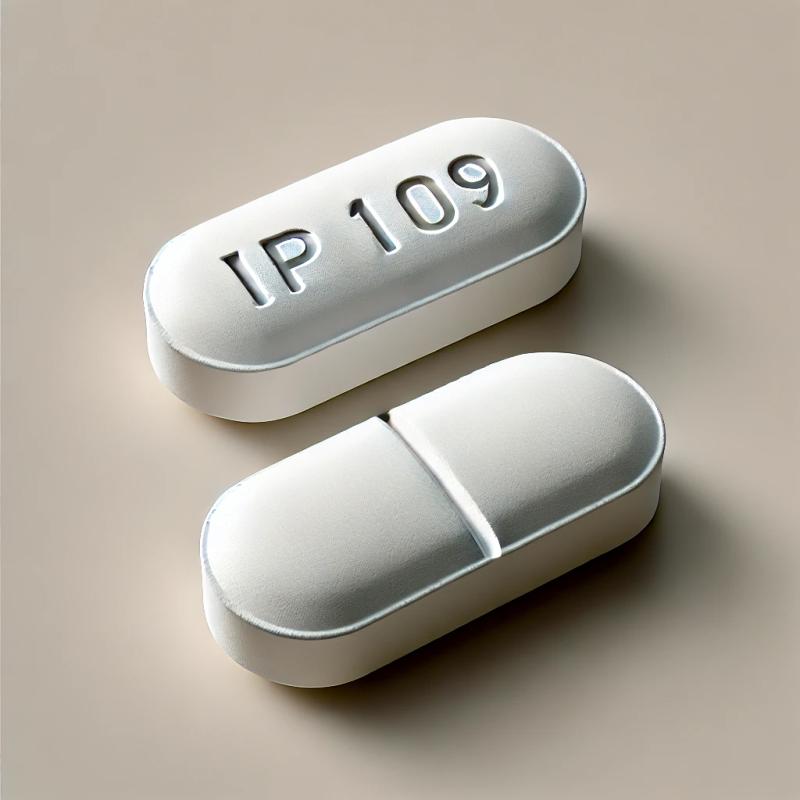
IP 109 pills are oval-shaped, white, and scored for easy splitting. The imprint "IP 109" is engraved on one side, helping to identify the medication. If you come across pills marked with "IP 109," ensure they are obtained from a legitimate prescription, as misuse or counterfeit versions can pose serious health risks. Always consult a healthcare professional if you are unsure about a pill’s identity.
- Imprint: IP 109
- Strength: 325 mg / 5 mg
- Color: White
- Size: 15.00 mm
- Shape: Oblong/Oval
Side effects and potential risks of IP 109
IP 109 is associated with side effects and risks that are similar to those of other opioids. Risks can range from mild to severe, depending on the dosage, duration of use, and patient or user-specific factors.
Common side effects
Common side effects associated with IP 109 involve the gastrointestinal tract and CNS. Which include: [5][6]
- Nausea and vomiting
- Constipation (opioid-induced)
- Drowsiness
- Dizziness
- Somnolence (sleepiness)
- Itching (pruritus)
Serious risks and warnings
Adverse effects that necessitate a warning include: [7][8][9]
- Respiratory depression: This can occur mainly when used in combination with other CNS depressants, like alcohol or benzodiazepines.
- Liver damage: Acetaminophen damages the liver at high doses. Exceeding 4,000 mg daily can cause severe liver injury, especially in chronic users, including those who consume alcohol.
- Addiction and dependence: This is due to hydrocodone's opioid properties.
Safe usage and precautions for IP 109
- Take only as prescribed: Use the exact dose your doctor recommends.
- Avoid alcohol: Drinking alcohol may increase the risk of severe liver damage and drowsiness.
- Watch out for overdose signs and symptoms: Symptoms like confusion, extreme sleepiness, or shallow breathing require immediate medical attention.
- Be careful when taking other medications: Don’t take other acetaminophen-containing drugs (like Tylenol), as this can lead to serious health complications and overdose.
- Don’t drive or use heavy machinery: IP 109 can make you sleepy or dizzy, which may lead to accidents or injury.
- Dependency risk: Hydrocodone is an opioid and can be addictive. Follow your doctor’s instructions to minimize this risk.
- Seek help if side effects are severe: Side effects, like nausea, confusion, or trouble breathing, mean you must call a doctor immediately.
IP 109 addiction and dependence potential
Hydrocodone is a key component of IP 109, and it is a driver of addiction, due to its effects on the brain's reward system. Hydrocodone binds to opioid receptors, which, in excess, may lead to feelings of euphoria and may help mitigate feelings of psychological distress. Feelings of euphoria and the use of IP 109 to cope with negative feelings can drive repeated or compulsive use and eventual dependence.
Below are some key symptoms and mechanisms linked to hydrocodone addiction:
- Euphoria and mood elevation: Hydrocodone enhances your mood and productivity, making people who use it feel energized and happy, which encourages repeated use. [10]
- Tolerance and dose escalation: Regular or frequent use leads to tolerance, requiring higher doses to achieve the same desirable effects–increasing the risk of addiction. [10]
- Withdrawal symptoms: Withdrawal symptoms, such as anxiety, depression, and flu-like symptoms, occur when IP 109 is stopped, driving people to resume its use to avoid the extreme discomfort of opioid withdrawal. [10]
- Compulsive drug-seeking behavior: People who use opioids may engage in behaviors, like doctor shopping or falsifying prescriptions, in order to obtain more hydrocodone. [11]
- Psychological dependence: People who use opioids often feel they need hydrocodone to function normally; in fact, that feeling is very real for them, even in the absence of physical pain.
- Neurological changes: Chronic hydrocodone use alters neurotransmitter systems, particularly glutamate and dopamine pathways, which exacerbate addiction and dependence. [12]
Can you overdose on IP 109?
Yes. Overdosing on IP 109 happens when you take a higher-than-prescribed dose or combine it with other substances, like alcohol, sedatives, or other acetaminophen-containing drugs.
Hydrocodone overdose symptoms (opioid component): [5][6]
- Extreme drowsiness or difficulty staying awake
- Slow or shallow breathing
- Pinpoint (constricted) pupils
- Cold, clammy skin
- Loss of consciousness or unresponsiveness
- Blue or pale lips and fingernails (cyanosis)
Acetaminophen overdose symptoms: [7][8]
- Nausea and vomiting
- Loss of appetite
- Abdominal pain, particularly in the upper right side (due to liver damage)
- Confusion or mental fog
- Jaundice (yellowing of skin and eyes, indicating liver damage)
- Dark urine
- Fatigue or extreme weakness
How to prevent an overdose
- Take the medication only as prescribed.
- Avoid mixing substances/drugs, otherwise known as polydrug use.
- Know your limits, as thresholds can vary by person.
Recognizing an IP 109 overdose emergency
An IP 109 overdose can become life-threatening, requiring immediate action. Key warning signs include:
- Shallow or stopped breathing.
- Unresponsiveness or unconsciousness.
- Severe liver damage symptoms such as jaundice (yellow skin or eyes), confusion, or dark urine.
- Persistent vomiting, particularly with signs of internal bleeding like dark blood in vomit or stool.
Emergency response for an IP 109 overdose
An overdose is an emergency that requires immediate medical attention. Swift action can save a life and mitigate long-term complications.
- Call emergency services: Dial 911 (or your local emergency number) immediately.
- Administer Naloxone: If available, use intranasal naloxone to counteract the effects of opioids.
- Do not induce vomiting: Do not attempt to make the person vomit, as this could worsen the situation.
- Ensure the person is breathing: If unconscious but breathing, position them on their left side (recovery position) to prevent choking. If they stop breathing, administer CPR if trained to do so.
- Provide medical information: Bring the IP 109 bottle or write down the dosage and usage details for healthcare professionals.
Alternatives to IP 109 for pain management
Different measures can be used to determine alternatives to IP 109, such as better or equal pain relief with fewer side effects, less potential for misuse and dependency, etc. However, this remains difficult, especially with the current opioid crisis, but pain should never be mismanaged or ignored at all. Some alternative medications for pain management include:
- Ibuprofen/acetaminophen combination: A fixed-dose combination of ibuprofen and acetaminophen provides greater pain relief than IP 109 and reduces the need for opioid rescue medication in acute pain management. [13]
- Naproxen sodium: In post-surgical dental pain, naproxen sodium has shown to be as effective as (and often superior to) IP 109 in reducing pain over 12 hours while having fewer side effects. [14]
- Celecoxib (COX-2 Inhibitor): Celecoxib provided comparable pain relief to IP 109 in orthopedic surgery patients, resulting in significantly fewer adverse events over five days. [15]
Opioid-sparing approaches
- Tramadol/acetaminophen: For musculoskeletal pain, tramadol-acetaminophen was found to be equally as effective as hydrocodone-acetaminophen (like IP 109). [16]
- Oxycodone/ibuprofen: A combination of oxycodone and ibuprofen outperformed both IP 109 and other hydrocodone-acetaminophen combinations (e.g., Vicodin, M367 pills) and oxycodone-acetaminophen combinations (e.g., Percocet) for dental pain, showing fewer opioid-related side effects. [17]
Treatment options for IP 109 misuse and addiction
If you or someone you know is struggling with addiction to the IP 109 hydrocodone pill, help is available. Harm reduction is one method, but treatment for hydrocodone addiction involves a multidisciplinary approach:
- Medical detoxification: Detox is always the first step in cases like these. During detox, the body is gradually weaned off the drug. This process, especially in the case of opioid use disorder, should be done under medical supervision because withdrawal symptoms can be intense, extremely uncomfortable, and distressing. Doctors will prescribe medications in some instances to help ease these symptoms and ensure the process is as safe and comfortable as possible.
- Inpatient rehab: Inpatient rehab programs provide a structured environment where people can focus entirely on recovery.
- Outpatient rehab: It allows people to stay home and travel to attend therapy sessions during weekdays or evenings. This type of rehab is ideal for people with a supportive home environment and milder addictions, or it may be suited for those who may not be able to afford inpatient care or rehab. People may also transition from inpatient to outpatient rehab.
- MAT (medication-assisted treatment): MAT involves using medications, like methadone or buprenorphine, to reduce cravings and withdrawal symptoms, especially for dependence on opioid medications. MAT is oftentimes combined with counseling and behavioral therapies as a holistic approach to treatment.
- Support groups: Support groups, like NA (Narcotics Anonymous), provide a community of people in recovery. Regular attendance of support groups has shown to be highly beneficial for maintaining long-term sobriety.
Treatment for addiction to IP 109 hydrocodone is a journey that requires commitment, but with the proper support and resources, recovery, better health, and improved well-being are possible. Visit our rehab directory to find a facility that can help you manage your addiction.

-person-thumbnail.jpg?v=1758880627)


-guide-detail.jpg?v=1756808659)